eSVS Animation
This high quality animation that depicts the use of the eSVS Mesh device and its related components.
Note: This animation is intended for educational puposes only. For full eSVS Mesh precribing information please click on the Instructions For Use tab above.
Angio Videos

76 year old male with a 3.5mm eSVS® Mesh to RCA

Control vs. eSVS Mesh at 9 Months (Human)

Control vs. eSVS Mesh at 9 Months (Human)

Angiographic Results Venous Graft Treated with eSVS Mesh
Same Patient at 10 and 24 Months
Same Patient at 10 and 24 Months

Angiographic Results Venous Graft Treated with eSVS Mesh
Same Patient at 9 and 36 Months
Same Patient at 9 and 36 Months

Angiographic Results Venous Graft Treated with eSVS Mesh
Same Patient at 9 and 36 Months
Same Patient at 9 and 36 Months
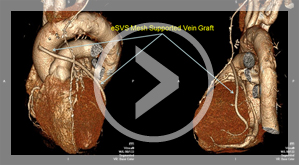
6 Months CT Scan (Human)
Clinical eSVS Mesh Prep

Short video demonstrating saphenous vein preparation and deployment of the eSVS Mesh in a clinical case at the Universitatsspital Basel in Switzerland.


